About Neurotrophic Keratitis
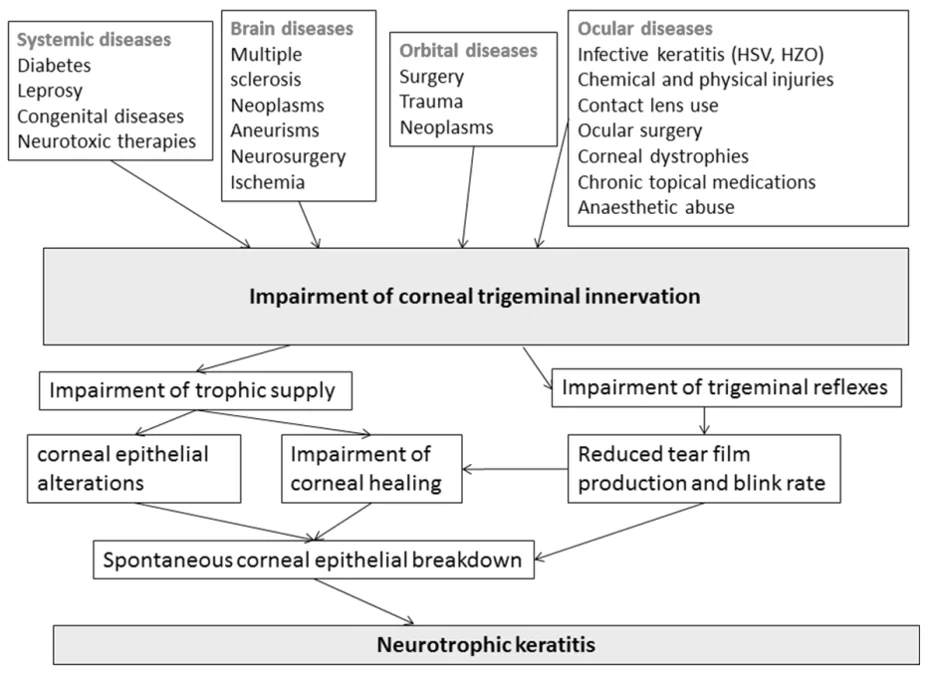
Neurotrophic Keratitis (NK) is a rare disease, with an estimated prevalence of less than 5 in 100,000. It is primarily caused by damage to the trigeminal nerve, which leads to reduced corneal sensitivity. Trigeminal nerve impairment disrupts sensory input to the cornea, reducing blink frequency and reflex tear secretion, ultimately resulting in persistent damage to the cornea.
The etiology of NK is multifactorial, and its clinical diagnosis remains challenging. Current treatment strategies for NK depend on the disease severity. Mild cases are usually managed with artificial tears or topical antibiotics to control inflammation and prevent infection. For moderate to severe cases , the orphan drug, Oxervate (cenegermin)—a recombinant human nerve growth factor that can promote corneal wound healing. An amniotic membrane device (Prokera) can also be used to protect the corneal surface and accelerate healing. If these treatments are ineffective, more invasive surgical procedures, such as tarsorrhaphy, conjunctival flap covering, amniotic membrane transplantation, or corneal transplantation, need to be considered.
Ref:modified from Mastropasqua, Massaro-Giordano et al. 2017
The impact of Neurotrophic Keratitis
Neurotrophic Keratitis can lead to permanent vision loss if not properly treated. It may result from any condition that affects the trigeminal nerve. Damage to the trigeminal nerve reduces corneal sensitivity, leading to decreased reflex tearing—the natural tear response to foreign particles such as dust. As corneal sensation decreases, reduced reflex tearing leads to epithelial dryness and eventual breakdown.

Affected individuals may experience frequent or recurrent corneal erosions (epithelial defects), which increase susceptibility to ocular infection.
More serious complications may develop, such as irregular astigmatism— a condition that causes blurred vision due to an irregularly shaped cornea. Affected individuals may develop corneal scarring. In severe cases, corneal perforation can occur,, resulting in the leakage of intraocular fluid.
BRM424
Preclinical studies have shown that BRM424 stimulates the proliferation and differentiation of limbal stem cells, effectively regenerating healthy limbal tissue and accelerating corneal repair even after extensive limbal excision. By directly activating limbal stem cells, BRM424 promotes rapid and effective corneal healing. The same short peptide has already demonstrated a favorable safety profile and early-onset efficacy in the clinical trials of BRM421, BRIM’s lead program for dry eye disease, further supporting the therapeutic potential of BRM424.
Compared with nerve growth factor-based or small-molecule therapies, BRM424 demonstrates a superior safety profile and has been granted Orphan Drug Designation (ODD) in the United States, offering regulatory advantages such as accelerated development pathways and market exclusivity. These benefits not only help shorten time-to-market and reduce development risks but also enhance overall competitiveness.
In a field where current treatment options for neurotrophic keratitis are limited and severe cases often require invasive surgery, BRM424 has the potential to provide patients with an effective, safe, and affordable non-invasive alternative.