
Business Model
BRIM translates research into clinical assets with the highest potential to positively impact patient lives. BRIM primarily targets highly novel projects with new molecular entities and niche platforms, targeting diseases where there are unmet medical needs. This involves, in-licensing from, or partnering with, academic institutions and biotech or pharma companies, both locally and internationally.
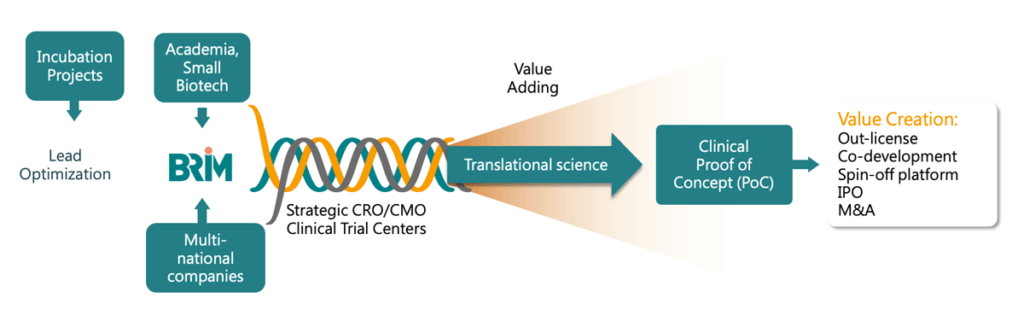
De-risking is the essence of value creation, delivered through our scientific expertise and our virtual team model. An internal integrated team of project management professionals and translational science experts provide expert input and operational leadership while outsourcing research activities to external entities such as research institutes, CROs, and CMOs.
BRIM’s model of connectivity, shared knowledge and scientific excellence means development times and costs are dramatically reduced. Upon reaching the clinical proof of concept (PoC), we out-license or partner with the best-suited third party to continue development through market approvals and commercialization, maximizing profit through royalties, milestones, or profit sharing. Our efficient business model delivers maximum ROI and is not limited by the traditional pharma R&D approach.
All drug development activities are executed according to global regulatory requirements and standards. Clinical trials are conducted to meet requirements for regulatory submissions to the US FDA through the help of CROs, CMOs, CDMOs, and partnership networks (e.g., hospitals, KOLs, government-sponsored agencies, research institutes,…etc.). BRIM’s translational science expertise enables rapid drug development and regulatory progression while ensuring high-quality standards.
